2023 — high level summary of research completed as part of MSc at Imperial College London
Background
Worldwide, the vast majority of hydrogen is produced via the steam methane reforming (SMR) and coal gasification (CG) processes. Due to the increasing concerns about man-made climate change, cleaner production options such as production from water electrolysis are also receiving increased attention. However, due to high cost of hydrogen produced from electrolysis the fraction of hydrogen produced via this production method worldwide is rather small (3.9%). To decrease the emissions from traditional SMR and CG, carbon capture systems are utilised, which is able to capture a significant percentage of CO2 from process and waste streams. H2 produced by these traditional methods coupled with a carbon capture system is considered blue hydrogen, and is seen as an enabler for transition to a decarbonised hydrogen industry.
What is Sorption Enhanced Hydrogen Production?
Sorption enhanced steam methane reforming (SE-SMR) shows promise as a method of in-situ CO2 capture within the SMR process using a solid sorbent.
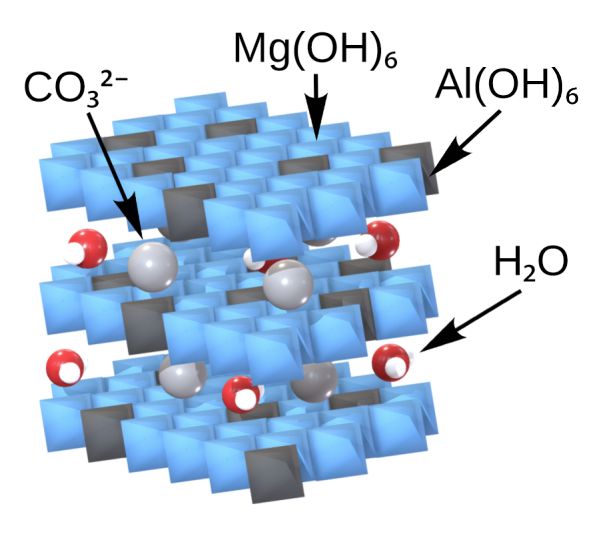
Calcium oxide, which occurs in nature as major component of Limestone, is a primary candidate for CO2 adsorption within the SE-SMR process. Lime, another form of CaO, has been used in the earliest SE-SMR experiments due to availability and well understood properties. Hydrotalcites (HTCs) represent another class of sorbent which has received significant attention for application within the SE-SMR process. They fall into the category of layered double hydroxides (LDHs). SE-SMR Processes based on each sorbent must take into account the individual properties when considering operating conditions and sorbent durability.
Potential benefits of SE-SMR over conventional steam reforming include:
- Reduced operating temperature
- Higher conversion possible
- Reduced purification and CO2 capture requirement
- Shift reactors no longer necessary
- Reduction in required heat exchange
equipment
SE-SMR is not a continuous process, rather it requires a combined adsorption/reaction step and a regeneration step. This can be performed cyclically in fixed beds as it done in Pressure Swing Adsorption, or in a moving bed configuration such as in a fluidised bed reactor.
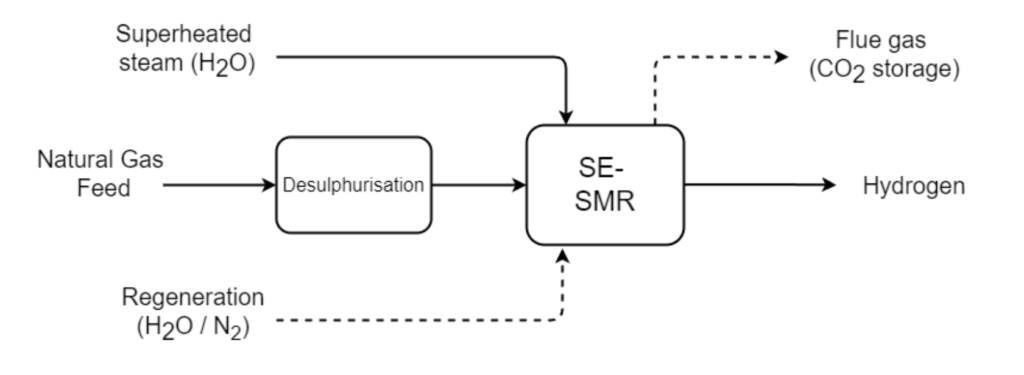
A two-step model simulating the isothermal adsorption of CO2 and H2O in a fixed bed using hydrotalcite (HTC) sorbent was developed as part of my Master of Science in Advanced Chemical Engineering at Imperial College London.
Research Summary
Four isotherms describing fixed-bed adsorption of CO2 on hydrotalcite were implemented in gPROMs Modelbuilder, notably including a multi-component isotherm which describes the competitive adsorption of H2O in addition to CO2 adsorption. Axial dispersion and a linear driving force model were also considered. A sensitivity study was performed to investigate model behaviour under relevant conditions. Effect of pressure, temperature, bed length, axial dispersion, and linear driving force on breakthrough profile, maximum adsorption capacity, and desorption profile have been investigated.
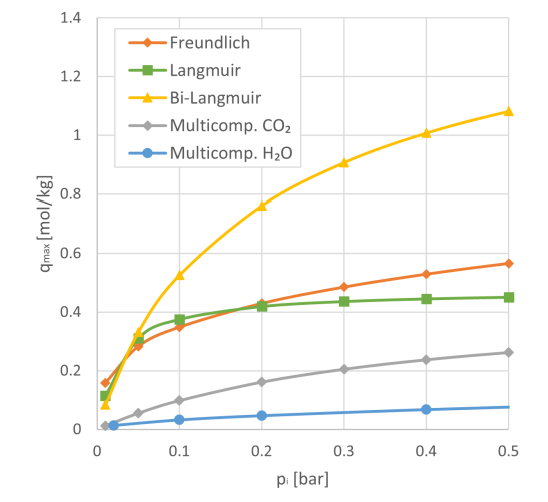


Key findings:
- kLDF below 0.05 shows significant oversmoothing of adsorption profile. H2O adsorption overshoot significantly increased by kLDF values above 0.1.
- Bed length strongly affects CO2 desorption profile (peaking).
- The investigated Freundlich, Langmuir & biLangmuir isotherms fail to describe adsorption behaviour accurately at higher partial pressures.
- The multicomponent isotherm can describe CO2 and H2O adsorption at pressures up to 20 bar, however shows lower capacities than the other investigated isotherms at lower partial pressures.
- Increase in pressure shows increase in CO2 and H2O capacities and decrease in breakthrough time, as well as increased competitive adsorption as nanopore regime dominates above 3 – 10 bar partial pressure.
- Increase in temperature produced a decrease in maximum adsorption capacity, where CO2 and H2O generally showed much higher capacities at low temperatures and are essentially surfaceadsorption limited above 550°C.
